Want to enroll?
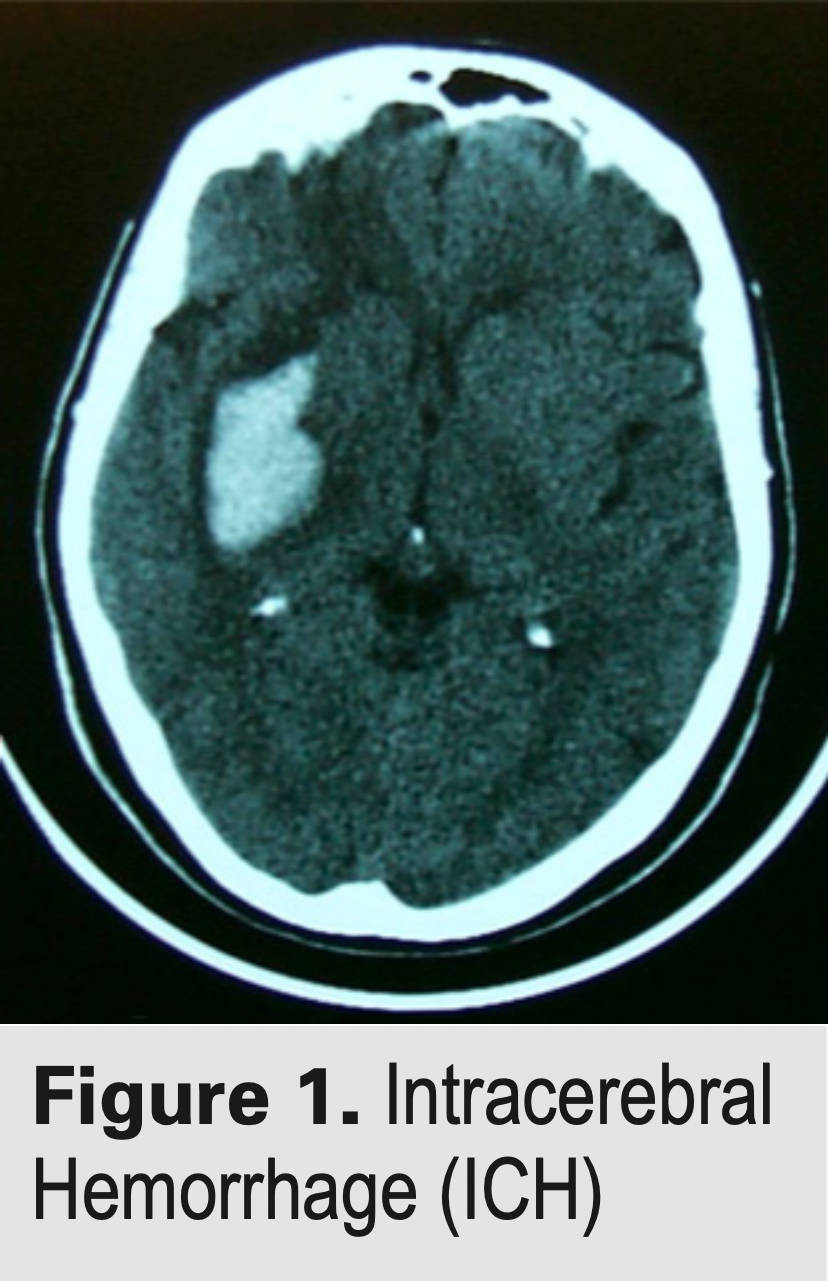
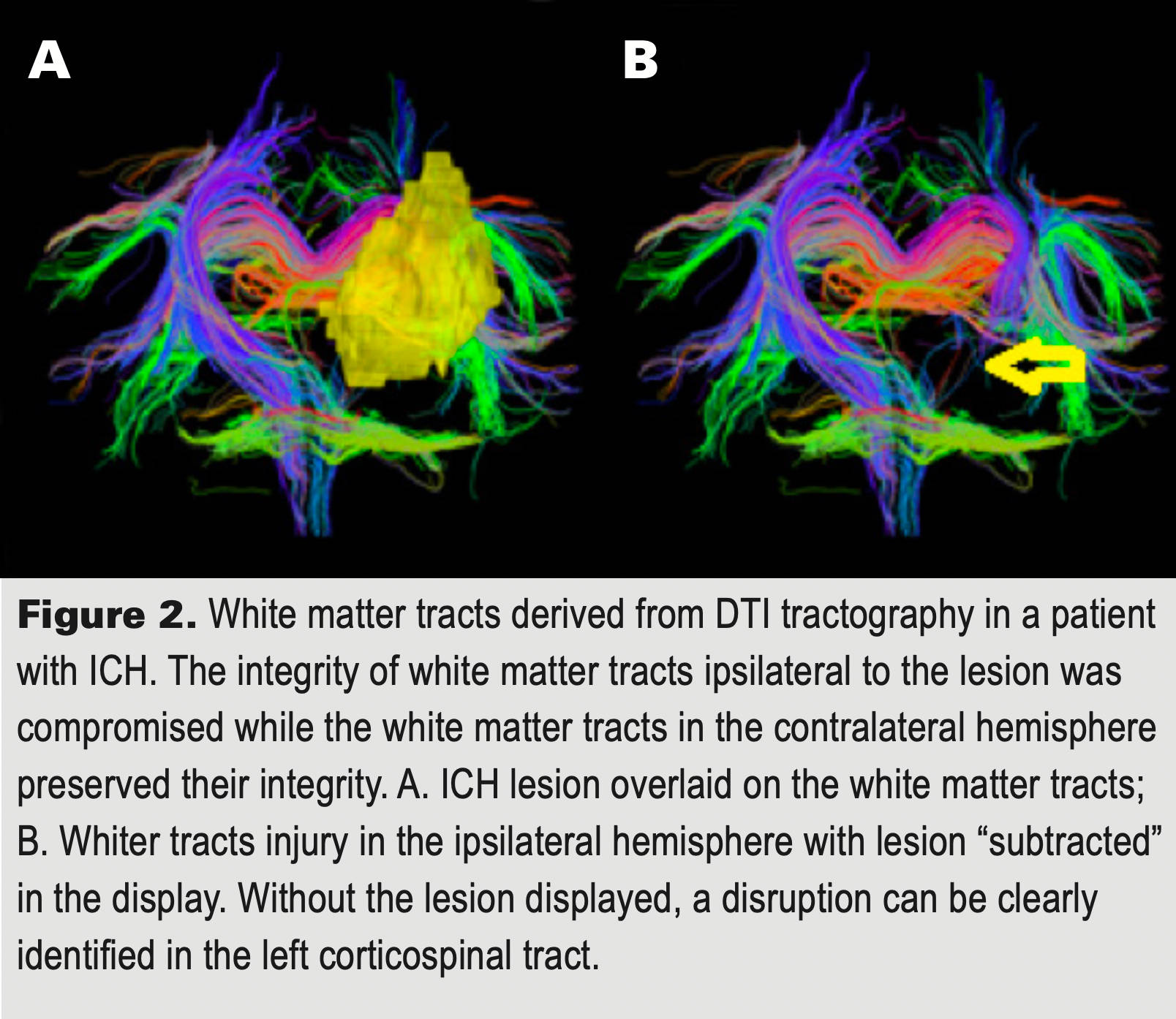
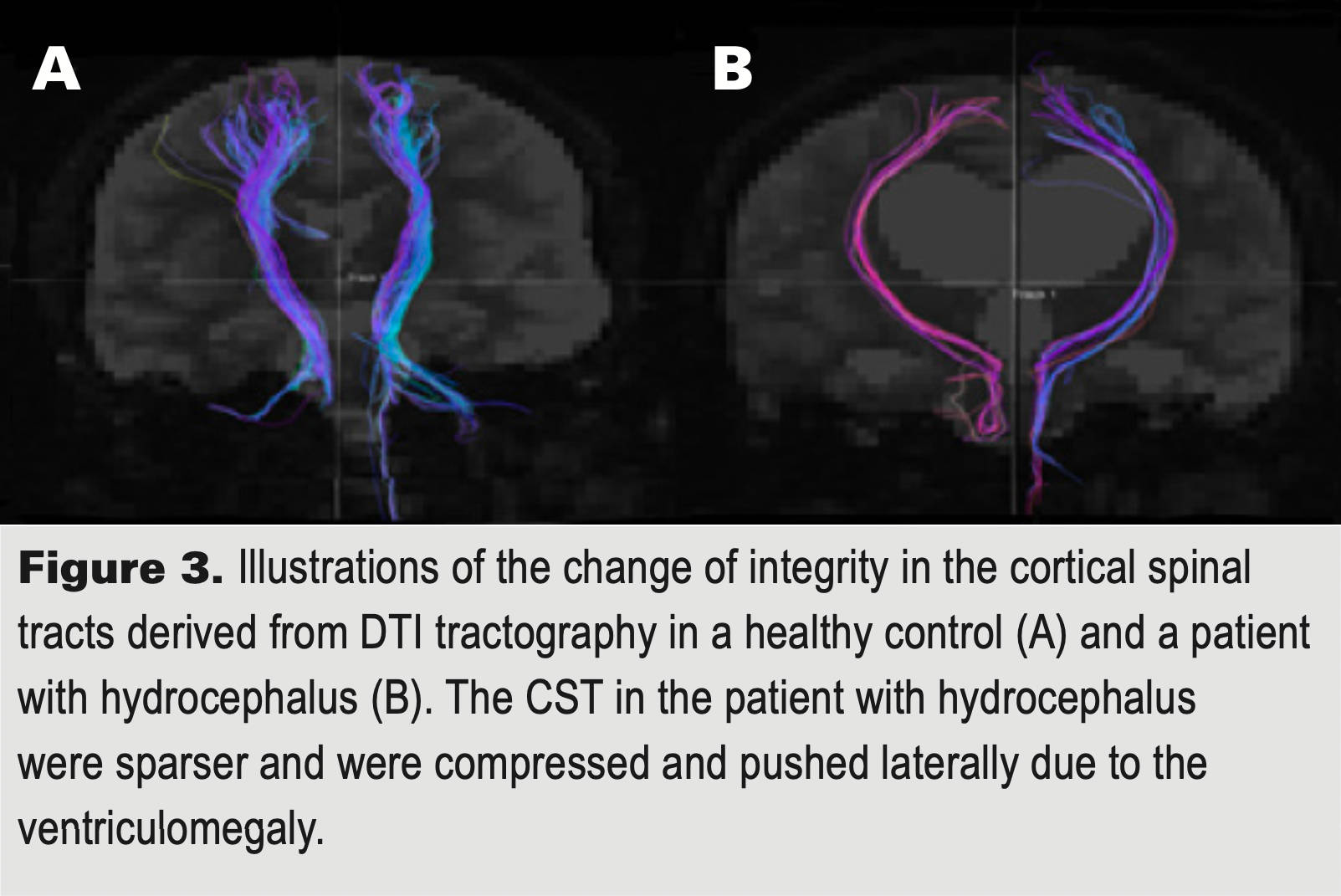
Because we need to perform analyses at roughly the time of their stroke, only those patients within 45 days of their hemorrhage are eligible for the study and there are other criteria for enrollment. If you are near one of the sites (see ‘Where is the study being conducted?), you may already have been approached by one of the investigators. If not, please feel free to contact us through the email below. Please leave as much information in the email as possible to be able to get back with you and in particular, what city you are in so that we can have the right center call you. Thank you for considering! With your help, we may be able to find new treatments for ICH!
Contact us